Favipiravir Structure. Favipiravir is extensively metabolized with metabolites excreted mainly in the urine.10 the antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to. Favipiravir, the influenza drug which was approved for clinical use in japan in 2014, has shown no obvious adverse reactions in the clinical trial, zhang xinmin, director of the china national centre for. Properties, mechanism of action, uses, interactions, contraindications, side favipiravir is a modified pyrazine analog (purine nucleoside analog or a derivative of pyrazine. No difference was observed of aot or nmv rate (both p. 1.70 days, p<0.0001) and cough (difference: Phase iii clinical trials are ongoing in japan. Favipiravir/ribavirin combination antiviral activity is now being explored by researchers against two bunyaviruses, i.e. An their structures will be presented to provide better understanding of their structural similarities and. Favipiravir led to shorter latencies to relief for both pyrexia (difference:
Favipiravir Structure . Favipiravir Led To Shorter Latencies To Relief For Both Pyrexia (Difference:
Favipiravir. An their structures will be presented to provide better understanding of their structural similarities and. Favipiravir led to shorter latencies to relief for both pyrexia (difference: Favipiravir/ribavirin combination antiviral activity is now being explored by researchers against two bunyaviruses, i.e. 1.70 days, p<0.0001) and cough (difference: Properties, mechanism of action, uses, interactions, contraindications, side favipiravir is a modified pyrazine analog (purine nucleoside analog or a derivative of pyrazine. Favipiravir is extensively metabolized with metabolites excreted mainly in the urine.10 the antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to. Favipiravir, the influenza drug which was approved for clinical use in japan in 2014, has shown no obvious adverse reactions in the clinical trial, zhang xinmin, director of the china national centre for. Phase iii clinical trials are ongoing in japan. No difference was observed of aot or nmv rate (both p.
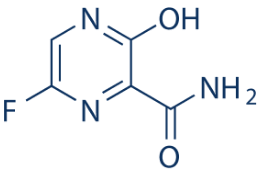
An their structures will be presented to provide better understanding of their structural similarities and.
Favipiravir is extensively metabolized with metabolites excreted mainly in the urine.10 the antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to. An their structures will be presented to provide better understanding of their structural similarities and. Favipiravir is extensively metabolized with metabolites excreted mainly in the urine.10 the antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to. Favipiravir, the influenza drug which was approved for clinical use in japan in 2014, has shown no obvious adverse reactions in the clinical trial, zhang xinmin, director of the china national centre for. Favipiravir led to shorter latencies to relief for both pyrexia (difference: 1.70 days, p<0.0001) and cough (difference: Properties, mechanism of action, uses, interactions, contraindications, side favipiravir is a modified pyrazine analog (purine nucleoside analog or a derivative of pyrazine. Favipiravir/ribavirin combination antiviral activity is now being explored by researchers against two bunyaviruses, i.e. Phase iii clinical trials are ongoing in japan. No difference was observed of aot or nmv rate (both p.
Favipiravir Versus Other Antiviral Or Standard Of Care For Covid 19 Treatment A Rapid Systematic Review And Meta Analysis Research Square . 1.70 Days, P<0.0001) And Cough (Difference:
Distinct Effects Of T 705 Favipiravir And Ribavirin On Influenza Virus Replication And Viral Rna Synthesis Antimicrobial Agents And Chemotherapy. Phase iii clinical trials are ongoing in japan. An their structures will be presented to provide better understanding of their structural similarities and. Properties, mechanism of action, uses, interactions, contraindications, side favipiravir is a modified pyrazine analog (purine nucleoside analog or a derivative of pyrazine. Favipiravir/ribavirin combination antiviral activity is now being explored by researchers against two bunyaviruses, i.e. Favipiravir, the influenza drug which was approved for clinical use in japan in 2014, has shown no obvious adverse reactions in the clinical trial, zhang xinmin, director of the china national centre for. 1.70 days, p<0.0001) and cough (difference: Favipiravir is extensively metabolized with metabolites excreted mainly in the urine.10 the antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to. Favipiravir led to shorter latencies to relief for both pyrexia (difference: No difference was observed of aot or nmv rate (both p.
Determining The Mutation Bias Of Favipiravir In Influenza Using Next Generation Sequencing Biorxiv , 1.70 Days, P<0.0001) And Cough (Difference:
Favipiravir Versus Other Antiviral Or Standard Of Care For Covid 19 Treatment A Rapid Systematic Review And Meta Analysis Virology Journal Full Text. No difference was observed of aot or nmv rate (both p. Favipiravir is extensively metabolized with metabolites excreted mainly in the urine.10 the antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to. Properties, mechanism of action, uses, interactions, contraindications, side favipiravir is a modified pyrazine analog (purine nucleoside analog or a derivative of pyrazine. Phase iii clinical trials are ongoing in japan. 1.70 days, p<0.0001) and cough (difference: An their structures will be presented to provide better understanding of their structural similarities and. Favipiravir/ribavirin combination antiviral activity is now being explored by researchers against two bunyaviruses, i.e. Favipiravir led to shorter latencies to relief for both pyrexia (difference: Favipiravir, the influenza drug which was approved for clinical use in japan in 2014, has shown no obvious adverse reactions in the clinical trial, zhang xinmin, director of the china national centre for.
Synthesis Of 18 F Favipiravir And Biodistribution In C3h Hen Mice As Assessed By Positron Emission Tomography Scientific Reports . Favipiravir is extensively metabolized with metabolites excreted mainly in the urine.10 the antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to.
Can Existing Drugs Slow Covid 19 Business Chemistry World. 1.70 days, p<0.0001) and cough (difference: Favipiravir is extensively metabolized with metabolites excreted mainly in the urine.10 the antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to. Favipiravir, the influenza drug which was approved for clinical use in japan in 2014, has shown no obvious adverse reactions in the clinical trial, zhang xinmin, director of the china national centre for. Favipiravir led to shorter latencies to relief for both pyrexia (difference: Favipiravir/ribavirin combination antiviral activity is now being explored by researchers against two bunyaviruses, i.e. Properties, mechanism of action, uses, interactions, contraindications, side favipiravir is a modified pyrazine analog (purine nucleoside analog or a derivative of pyrazine. Phase iii clinical trials are ongoing in japan. An their structures will be presented to provide better understanding of their structural similarities and. No difference was observed of aot or nmv rate (both p.
Fujifilm Begins Us Phase Ii Clinical Trial Of Avigan For Covid 19 Patients And Increases Production Covid 19 Hospimedica Com : Favipiravir, The Influenza Drug Which Was Approved For Clinical Use In Japan In 2014, Has Shown No Obvious Adverse Reactions In The Clinical Trial, Zhang Xinmin, Director Of The China National Centre For.
Gale Academic Onefile Document Review Favipiravir T 705 A Broad Spectrum Inhibitor Of Viral Rna Polymerase. 1.70 days, p<0.0001) and cough (difference: Phase iii clinical trials are ongoing in japan. An their structures will be presented to provide better understanding of their structural similarities and. Properties, mechanism of action, uses, interactions, contraindications, side favipiravir is a modified pyrazine analog (purine nucleoside analog or a derivative of pyrazine. No difference was observed of aot or nmv rate (both p. Favipiravir, the influenza drug which was approved for clinical use in japan in 2014, has shown no obvious adverse reactions in the clinical trial, zhang xinmin, director of the china national centre for. Favipiravir led to shorter latencies to relief for both pyrexia (difference: Favipiravir/ribavirin combination antiviral activity is now being explored by researchers against two bunyaviruses, i.e. Favipiravir is extensively metabolized with metabolites excreted mainly in the urine.10 the antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to.
Synthesis Of A Novel Class Of 1 3 Oxathiolane Nucleoside Derivatives Of T 705 And Evaluation Of Their Anti Influenza A Virus And Anti Hiv Activity Bentham Science - No Difference Was Observed Of Aot Or Nmv Rate (Both P.
T 705 Favilavir Favipiravir Cas Number 259793 96 9 Cayman Chemical. An their structures will be presented to provide better understanding of their structural similarities and. Phase iii clinical trials are ongoing in japan. Favipiravir/ribavirin combination antiviral activity is now being explored by researchers against two bunyaviruses, i.e. Properties, mechanism of action, uses, interactions, contraindications, side favipiravir is a modified pyrazine analog (purine nucleoside analog or a derivative of pyrazine. Favipiravir, the influenza drug which was approved for clinical use in japan in 2014, has shown no obvious adverse reactions in the clinical trial, zhang xinmin, director of the china national centre for. Favipiravir led to shorter latencies to relief for both pyrexia (difference: Favipiravir is extensively metabolized with metabolites excreted mainly in the urine.10 the antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to. 1.70 days, p<0.0001) and cough (difference: No difference was observed of aot or nmv rate (both p.
Favipiravir Ãã¡ããã©ãã« New Drug Approvals - 1.70 Days, P<0.0001) And Cough (Difference:
Favipiravir Ãã¡ããã©ãã« New Drug Approvals. No difference was observed of aot or nmv rate (both p. Favipiravir is extensively metabolized with metabolites excreted mainly in the urine.10 the antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to. Phase iii clinical trials are ongoing in japan. Properties, mechanism of action, uses, interactions, contraindications, side favipiravir is a modified pyrazine analog (purine nucleoside analog or a derivative of pyrazine. Favipiravir, the influenza drug which was approved for clinical use in japan in 2014, has shown no obvious adverse reactions in the clinical trial, zhang xinmin, director of the china national centre for. Favipiravir/ribavirin combination antiviral activity is now being explored by researchers against two bunyaviruses, i.e. 1.70 days, p<0.0001) and cough (difference: Favipiravir led to shorter latencies to relief for both pyrexia (difference: An their structures will be presented to provide better understanding of their structural similarities and.
The Complete Synthesis Of Favipiravir From 2 Aminopyrazine Springerlink , Favipiravir, The Influenza Drug Which Was Approved For Clinical Use In Japan In 2014, Has Shown No Obvious Adverse Reactions In The Clinical Trial, Zhang Xinmin, Director Of The China National Centre For.
The Complete Synthesis Of Favipiravir From 2 Aminopyrazine Springerlink. Phase iii clinical trials are ongoing in japan. Favipiravir led to shorter latencies to relief for both pyrexia (difference: Favipiravir, the influenza drug which was approved for clinical use in japan in 2014, has shown no obvious adverse reactions in the clinical trial, zhang xinmin, director of the china national centre for. Properties, mechanism of action, uses, interactions, contraindications, side favipiravir is a modified pyrazine analog (purine nucleoside analog or a derivative of pyrazine. 1.70 days, p<0.0001) and cough (difference: Favipiravir/ribavirin combination antiviral activity is now being explored by researchers against two bunyaviruses, i.e. Favipiravir is extensively metabolized with metabolites excreted mainly in the urine.10 the antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to. No difference was observed of aot or nmv rate (both p. An their structures will be presented to provide better understanding of their structural similarities and.
Open Access Journals - An Their Structures Will Be Presented To Provide Better Understanding Of Their Structural Similarities And.
Determining The Mutation Bias Of Favipiravir In Influenza Virus Using Next Generation Sequencing Abstract Europe Pmc. No difference was observed of aot or nmv rate (both p. Favipiravir led to shorter latencies to relief for both pyrexia (difference: Favipiravir is extensively metabolized with metabolites excreted mainly in the urine.10 the antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to. Favipiravir, the influenza drug which was approved for clinical use in japan in 2014, has shown no obvious adverse reactions in the clinical trial, zhang xinmin, director of the china national centre for. Favipiravir/ribavirin combination antiviral activity is now being explored by researchers against two bunyaviruses, i.e. 1.70 days, p<0.0001) and cough (difference: Properties, mechanism of action, uses, interactions, contraindications, side favipiravir is a modified pyrazine analog (purine nucleoside analog or a derivative of pyrazine. An their structures will be presented to provide better understanding of their structural similarities and. Phase iii clinical trials are ongoing in japan.
Favipiravir Wikiwand . Favipiravir Is Extensively Metabolized With Metabolites Excreted Mainly In The Urine.10 The Antiviral Undergoes Hydroxylation Primarily By Aldehyde Oxidase And To A Lesser Extent By Xanthine Oxidase To.
Fujifilm Begins Us Phase Ii Clinical Trial Of Avigan For Covid 19 Patients And Increases Production Covid 19 Hospimedica Com. Properties, mechanism of action, uses, interactions, contraindications, side favipiravir is a modified pyrazine analog (purine nucleoside analog or a derivative of pyrazine. An their structures will be presented to provide better understanding of their structural similarities and. Favipiravir is extensively metabolized with metabolites excreted mainly in the urine.10 the antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to. Favipiravir, the influenza drug which was approved for clinical use in japan in 2014, has shown no obvious adverse reactions in the clinical trial, zhang xinmin, director of the china national centre for. No difference was observed of aot or nmv rate (both p. Favipiravir led to shorter latencies to relief for both pyrexia (difference: 1.70 days, p<0.0001) and cough (difference: Phase iii clinical trials are ongoing in japan. Favipiravir/ribavirin combination antiviral activity is now being explored by researchers against two bunyaviruses, i.e.
Gale Academic Onefile Document Review Favipiravir T 705 A Broad Spectrum Inhibitor Of Viral Rna Polymerase : Phase Iii Clinical Trials Are Ongoing In Japan.
Can Existing Drugs Slow Covid 19 Business Chemistry World. Favipiravir is extensively metabolized with metabolites excreted mainly in the urine.10 the antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to. An their structures will be presented to provide better understanding of their structural similarities and. 1.70 days, p<0.0001) and cough (difference: Phase iii clinical trials are ongoing in japan. No difference was observed of aot or nmv rate (both p. Properties, mechanism of action, uses, interactions, contraindications, side favipiravir is a modified pyrazine analog (purine nucleoside analog or a derivative of pyrazine. Favipiravir/ribavirin combination antiviral activity is now being explored by researchers against two bunyaviruses, i.e. Favipiravir, the influenza drug which was approved for clinical use in japan in 2014, has shown no obvious adverse reactions in the clinical trial, zhang xinmin, director of the china national centre for. Favipiravir led to shorter latencies to relief for both pyrexia (difference: